In order to understand and treat diseases, we need to study them. Researchers sometimes face challenges in collecting samples of diseased tissues to study. To overcome this, researchers may develop ‘models’ of the disease. These are cells or tissues grown in the lab which imitate the behaviour of the disease in the body. Stem cells, including 'reprogrammed’ induced pluripotent stem cells (iPS cells), are an important source of cells for creating these disease models. What does it mean to ‘reprogram’ a cell, and why is this so important in disease modelling?
What do we know?
In the body, the process of cellular differentiation cannot be reversed. However, scientists are able to reverse this process in the lab, turning adult cells back into stem cells. These cells (‘induced pluripotent stem cells’ or iPS cells) can then be used to make any type of cell found in the body, such as heart or brain cells. This process is called ‘reprogramming’.
Because iPS cells have the same genes and mutations as the patient they come from, researchers can use iPS cells to recreate diseases in the lab and study how a patient’s genetics contribute to their disease.
iPS cells allow researchers to observe and study ‘cell differentiation’, the process of cells becoming specialised, and what can go wrong during differentiation to cause different diseases.
What are researchers working on?
Many diseases are now being studied using iPS cell model systems. These span from from neurological diseases to blood and immunodeficiency conditions.
iPS cells are being used to create disease-type cells and tissues for testing new drugs and treatments in the lab.
Researchers are using iPS cells to ‘turn back the clock’ on patients’ cells and watch how healthy cells become diseased, or to compared diseased cells with healthy cells.
iPS cells also let researchers examine how patients’ genes, mutations and environmental conditions might impact disease progression.
What are the challenges?
Researchers are still learning about iPS cells. In theory, iPS cells can make any cell in the body. However, researchers must first learn how to direct their differentiation down different pathways.
iPS cells can provide researchers with cells that contain the genes and mutations associated with a disease. This does not mean specialised cells created in the lab from iPS cells will develop and behave the same way diseased cells in the body do.
Modelling complex diseases (e.g. those caused by problems occurring between cells that make up complex structures, tissues and organs) with iPS cells is currently not possible. It may become possible in the future.
What is disease modelling?
Human diseases are many and varied. Whether a disease can be treated often depends on whether we can gain a good understanding of its basic biology. Without doing research to uncover how a disease works, it is difficult to advance treatments.
Disease modelling allows scientists to explore how a disease works in the laboratory, rather than directly in a patient. A disease model is a representation of the abnormal human or animal biology that occurs in a particular disease.
The model may be an animal with a condition that mimics a human disease, or it could be cells in a dish. Whatever the model, it must reproduce aspects of a disease, or even the whole disease pathology (all the physical effects of the disease), outside the human body.
Animal models in disease research
Animal models, such as mice, are widely used in research. They have been studied for many years and can develop symptoms similar to human disease symptoms. However, animals can never fully recreate all aspects of human biology or disease. Treatments that have been developed and found to be effective in experimental animal models can give vital clues and information, but do not always work when transferred to human patients.
Animal experimentation has other limitations. Ethical considerations mean that animals should be used in research only when it is essential to answer the research question. Developing animal models for a particular disease is also time-consuming.
Disease models based on human cells can help address these problems. Using cells removes the question of differences between animals and humans, because scientists can study human cells.
Human cells in disease modelling
Human cells were first cultured in the laboratory in the 19th century. Since then, our understanding of cells has improved greatly. Technological advances have made it easier to keep human cells alive, healthy and free from contamination outside the protection of the body.
Many of these advances come from research using cancer cells. Cancer cells can grow for a prolonged time - even indefinitely, if given the right environment. Healthy cells have a limited lifespan and are much more difficult to maintain and multiply in a dish.
Healthy cells can also be difficult to collect for research. Some cells, such as skin cells or blood cells, can be taken from a patient relatively easily. Others are far more difficult or even impossible to obtain for research. For example, human brain cells are difficult to extract without major surgery. However, they are needed to study a large number of neurodegenerative diseases. Certain types of stem cells may offer a solution.
How can stem cells help with disease modelling?
Stem cells can both self-renew (copy themselves) and differentiate (change) into more specialised cells. One particular type of stem cell offers a powerful tool for disease modelling: induced pluripotent stem cells, or iPS cells.
These lab-grown stem cells are made by ‘reprogramming’ specialised cells such as skin cells. This reverses the differentiation process, and restores them to a stem cell state. The resulting iPS cells can produce all the different types of cells in the body. This means they provide source of cells that are otherwise difficult to obtain, such as brain cells. Scientists are working on methods for directing iPS cells to make a specific type of cell on demand.
Embryonic stem cells, obtained from early stage embryos, also have the ability to make all the different cells of the body. However, iPS cells have a unique advantage for disease modelling: they can be made by reprogramming a patient’s own skin cells to make patient-specific cells in the laboratory. If the disease has a genetic cause, the lab-grown cells will carry that genetic flaw.
iPS cells have another benefit for researchers. Diseases are often detected when symptoms appear. This may be long after the disease process began in the body. This makes it almost impossible to study the early stages of certain diseases. Reprogramming technology acts like a time machine, taking mature cells like skin cells and returning them to their earlier, embryonic state. These embryonic-like iPS cells could then be used to make any cells the researcher wants to study, from early to late stages of cell development. This gives scientists a tool to model events in the diseased human body. Cells made in this way are called iPS cell disease models.
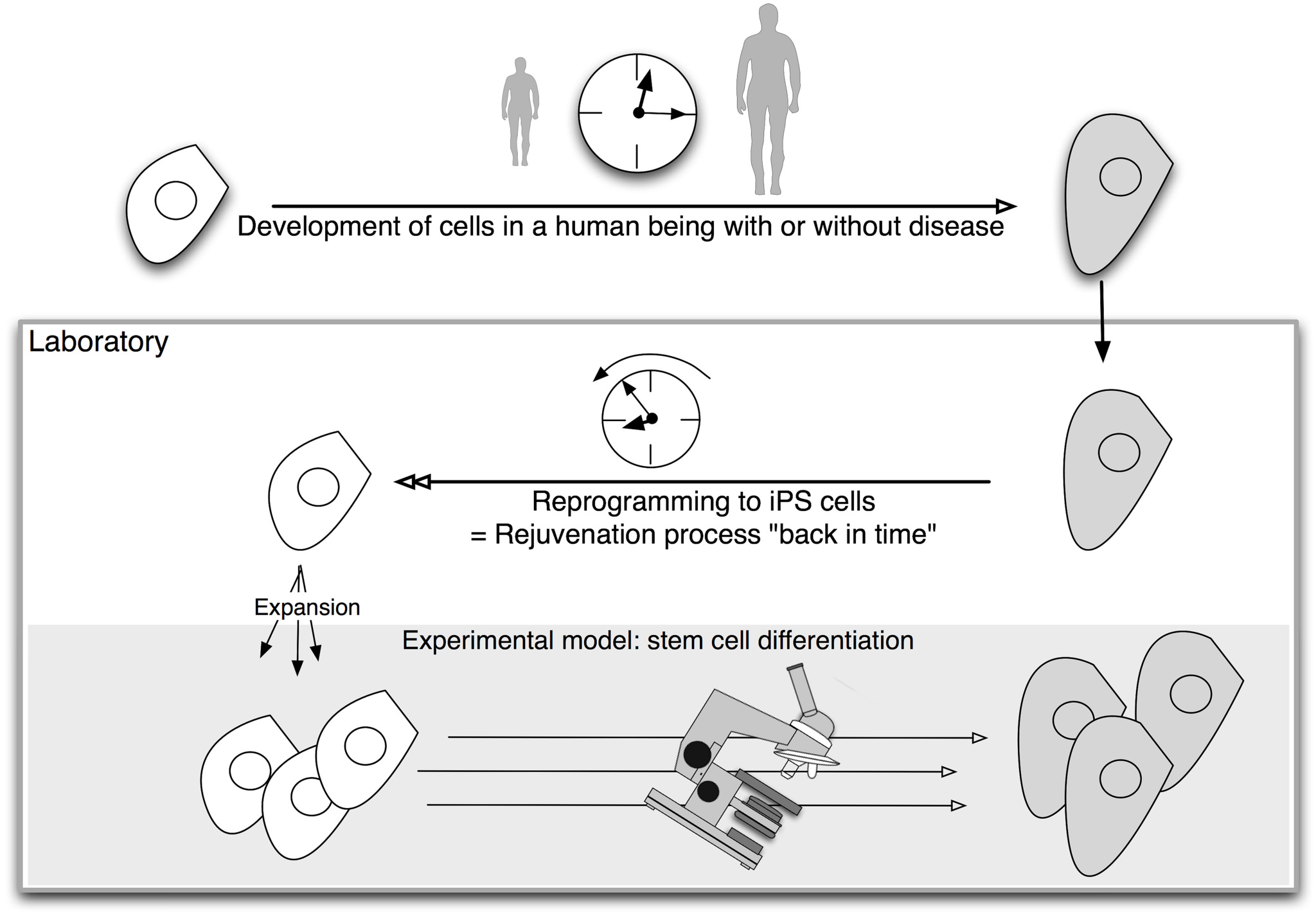
The use of iPS cells for disease modelling
In the last few years iPS cells have been created from patients with a number of different diseases, including:
- Spinal muscular atrophy (SMA)
- Motor neurone disease (MND) (also called amyotrophic lateral sclerosis (ALS) and Lou Gehrig’s Disease)
- Alzheimer’s Disease
- Parkinson’s Disease
- Huntington’s Disease
- Down Syndrome (also called Down’s Syndrome)
- Ataxia Telangiectasia (AT) also known as Louis-Bar Syndrome, immunodeficiency with ataxia telangiectasia, and cerebello-occulocutaneous telangiectasia)
- Friedreich’s Ataxia
Disease models using iPS cells are also under development for many other conditions, including neurological, blood-related, metabolic, cardiovascular, immunodeficiency and other conditions. The number of diseases studied in this way is increasing all the time.
Challenges in disease modelling
iPS cells have been widely adopted for disease modelling.They also carry certain chalalenges, and they may not be the right tool to answer all research questions.
- Understanding iPSCs: iPS cells are a completely new, lab-made type of stem cell. More research is needed to understand the reprogramming process and its effects on the cells. We also need to evaluate their potential uses and risks.
- Recreating a disease is complex: The aim of disease modelling is to use iPS cells to accurately recreate features of a disease outside the body. This is done by generating cells with a patient’s genetic code. However, this does not always result in cells with characteristics of the disease. Although diseases are often fully or partially caused by genetics errors, other biological processes also affect or initiate many diseases. Some of these disease processes cannot be easily recreated with current iPS cell technology. However, iPS cell disease modelling can help researchers to investigate how much of a disease is caused by DNA changes.
- Modelling diseases with different causes: Although iPS cells can theoretically be used to model any disease, they are more suitable for modelling diseases caused by mutations in a single gene (monogenic diseases) than for modelling diseases caused by alterations of several genes (polygenic or complex diseases) or diseases caused by environmental factors.
- Diseases often affect many types of cells: iPS cell disease models allow researchers to study how specific types of cells are affected by a disease. However, diseases usually affect complex tissues or organs, containing several kinds of cells in many interacting layers. The interaction between different cells often contributes to the effects of a disease. Researchers are building more complex structures, incorporating several cell types to model these aspects of diseases more completely. One approach is to use stem cells to grow groups of cells in 3D structures that resemble the tissue of different organs. These 3D structures offer more complex disease models than a flat (2D) cell culture. Examples of this approach include organoids and tissue engineered structures. Such ‘organoids’ offer more complex disease models than flat (2D) cell culture.
Develping future therapies
iPS cells give researchers the opportunity to study previously inaccessible human cells, such as the nerve cells of the brain. Their use in disease models provides a new stepping-stone towards a better understanding of how many diseases work. A great deal of research using iPS cells is already underway and researchers expect that iPS cell disease models will provide valuable clues for the development of new medicines or other clinical treatments.
