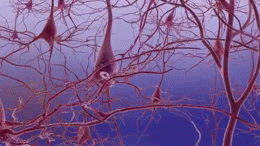
Alzheimer’s disease is the most common cause of dementia. It is a complex disease that affects nerve cells in many parts of the brain, making effective treatment very challenging. Can gene and cell therapy techniques help us tackle this challenge in the future?
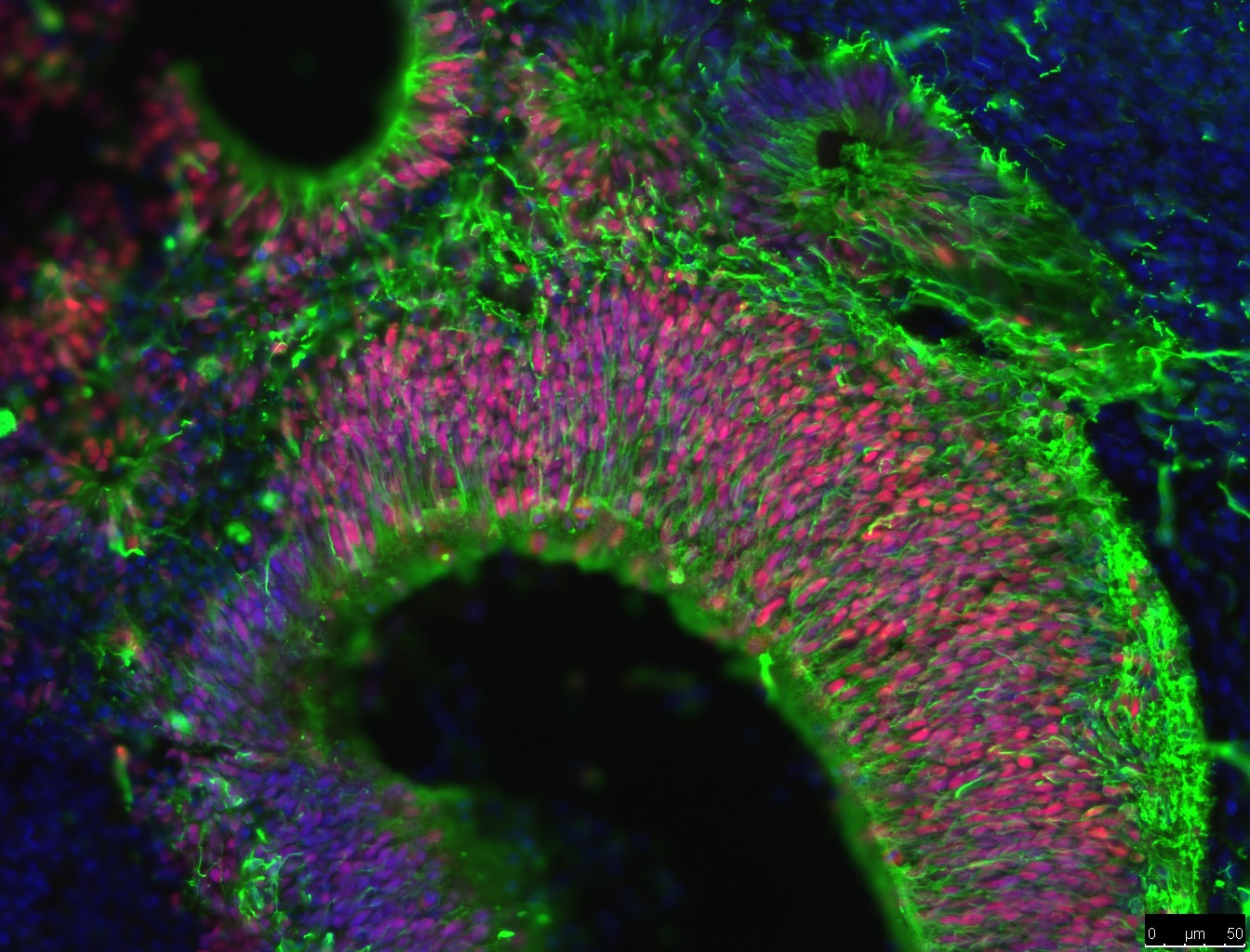
Researchers are using a type of stem cell called induced pluripotent stem cells (iPSCs) to study AD. These lab-grown stem cells are made by ‘reprogramming’ other cell types easily taken from patients, such as skin cells. The resulting iPS cells can produce all the different types of cells in the body. This means they could act as a source of cells that are otherwise difficult to obtain, such as the neurons found in the brain.
Scientists are using iPSC technology to grow neurons in the lab to study AD. Researchers use iPSC-derived neurons created from the cells of people with ADto study any abnormalities that might promote the progression of Alzheimer’s disease. This includes studying differences in how these neurons produce, move and release the amyloid beta protein (which forms plaques), and tau protein (which forms the tangles in patients’ brains).
Neurons derived from iPSCs give scientists a valuable opportunity to study neurons similar to the neurons in the brains of people with AD in the laboratory, offering a level of detail that would otherwise not be possible. This allows researchers to gain a much better understanding of how and why protein plaques are formed at the very start of the disease, and what causes neurons to die. It lets researchers experiment with new drugs and therapeutic approaches, and provides a tool to hunt for signposts that can help diagnose AD earlier, increasing the chance of success for new treatments.