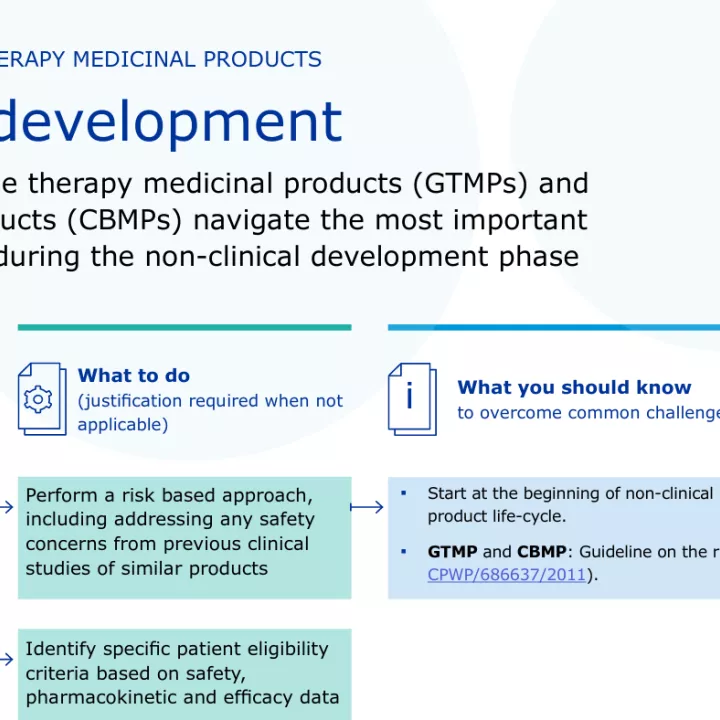
European Medicines Agency (EMA) has published a guide to help gene and cell therapy developers navigate the most important regulatory requirements during the non-clinical development phase.
Did you find the content on this page useful? If not, you can leave us a message so we can improve Send us your thoughts