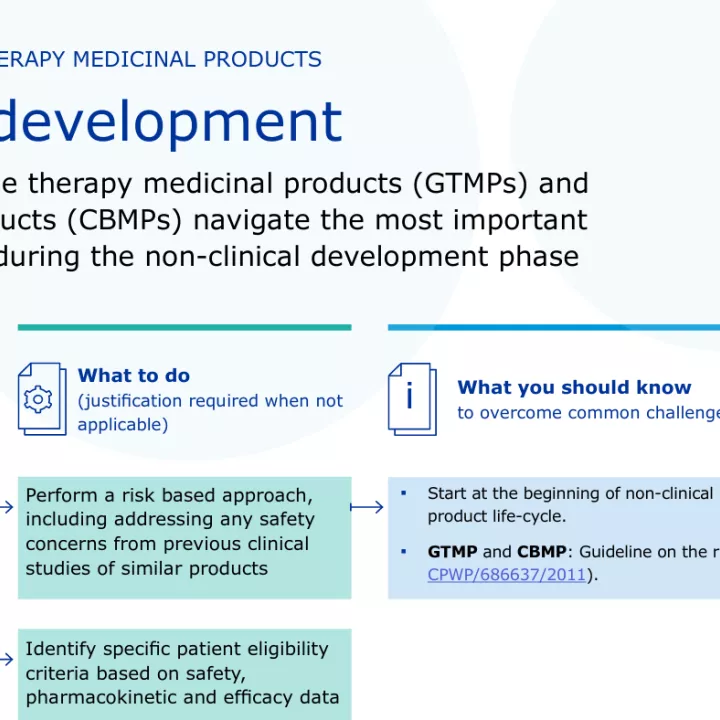
European Medicines Agency (EMA) has published a guide to help gene and cell therapy developers navigate the most important regulatory requirements during the non-clinical development phase.
Hai trovato le informazioni in questa pagina utili? In caso contrario puoi lasciarci un messaggio per aiutarci a migliorare Mandaci i tuoi commenti