MSCs: what are they and what can they be used for?
MSCs (also known as Mesenchymal Stem Cells, Mesenchymal Stromal Cells, or Medicinal Signalling Cells) are non-haematopoietic (non-blood), self-renewable cells. They can make several types of cells belonging to our skeletal tissues, such as cartilage, bone and fat. They also release a wide range of signalling molecules which have been shown to have influence the immune system and the body’s repair systems. Scientists are currently investigating how MSCs and their released molecules can be used to treat a range of conditions.
What do we know?
MSCs (also known as (Mesenchymal Stem Cells, Mesenchymal Stromal Cells or Medicinal Signalling Cells) constitute a specific adult tissue stem cell population. They were first isolated in the bone marrow, where they are important for creating a suitable ‘niche’ or environment for blood stem cells (haematopoietic stem cells). They also play an important role making and repairing skeletal tissues, such as cartilage, bone, and fat.
MSCs make up a very small fraction of all the cells in our bone marrow. However, thanks to their ability to self-renew, researchers have been able to isolate and grow these cells in the laboratory so they can be further studied. While MSCs were firstly isolated from bone marrow, scientists have currently been able to successfully isolate MSC-like cells from other adult tissues such as adipose (fat) tissue and neonatal tissues (such as umbilical cord or placenta tissues).
Another characteristic of these cells is that they release a wide range of signalling molecules, which communicate with other cells in the body. These molecules have been shown to have immunomodulatory, anti-inflammatory and pro-regenerative properties. Therefore, scientists are currently studying the use MSCs and their secreted molecules as potential novel therapies for treating many conditions. These include immune-mediated disorders as well as chronic diseases.
What are researchers working on?
MSC treatments are being developed to help repair bone and cartilage, such as injuries to the knee meniscus or long-term accumulated damage that leads to osteoarthritis.
Other studies are investigating whether MSCs may help new blood vessels form in damaged tissue. This could have major implications for fixing tissue damaged by heart attacks and other diseases such as peripheral arterial diseases or non-healing diabetic foot ulcers.
Researchers are also examining the ability of MSCs to reduce inflammation, slow the progression of autoimmune diseases and prevent transplant rejection.
What are the challenges?
Stem cell research is complex, detailed, slow and difficult. Conflicting results in early (and present day) MSC research are a reminder that stem cell research takes time to get right.
There is still a large amount of uncertainty in how MSCs can be successfully delivered to damaged tissues in the body. Often, transplanted MSCs are rapidly removed from the patient’s body by the immune system, or fail to integrate into the tissue. This may limit their ability to be used for treatments.
Some scientists are working to develop ways of holding MSCs in place: for instance, using biomaterials that support cell viability and retention into the transplanted site, so transplanted cells can live longer. In addition, the current mechanism of action of these cells (i.e. what exactly is causing the therapeutic benefit in the patient) is not yet fully understood.
The poor rate of engraftment of transplanted MSCs into the patients’ own tissue has led scientists to believe that this benefit does not come from the differentiation of MSCs into other cell types. Rather, this benefit may be due the signalling molecules secreted by MSCs to which may have effects in other cell types in our body contributing to tissue regeneration and repair. Other scientists have discovered that a large proportion of transplanted MSCs die after being delivered into the body. These dead cells are swallowed by specific immune cells called macrophages, which respond by helping to reduce inflammation and contribute to tissue repair and regeneration.
What can MSCs do?
MSCs (also known as Mesenchymal Stem Cells, Mesenchymal Stromal Cells or Medicinal Signalling Cells) are an example of adult tissue or 'adult' stem cells.
MSCs have the ability to self-renew. This means that they can multiply from one single cell to millions of cells using cell culture flasks in the laboratory (Fig. 1A). Collecting large numbers of MSCs from a patient or donor is challenging. This ability to expand into large number of cells in the laboratory is what allows scientists and clinicians to obtain number large enough to use for therapies.
MSCs are multipotent. This means they can produce more than one type of specialised cell of the body, but not all types. MSCs make different specialised cells found in the skeletal tissues. For example, they can specialise (differentiate) into fat cells (or adipocytes) (Fig. 1B), bone cells (or osteocytes) (Fig. 1C) and cartilage cells (or chondrocytes) (Fig. 1D). These specialised cells each have their own characteristic shapes, structures and functions, and each belongs in a particular tissue.
Some early research suggested that MSCs might also differentiate into other different types of cells that do not belong to the skeletal tissues, such as nerve cells, heart muscle cells, liver cells and endothelial cells (cells that form the inner layer of blood vessels). However, these results have not been confirmed in later studies.
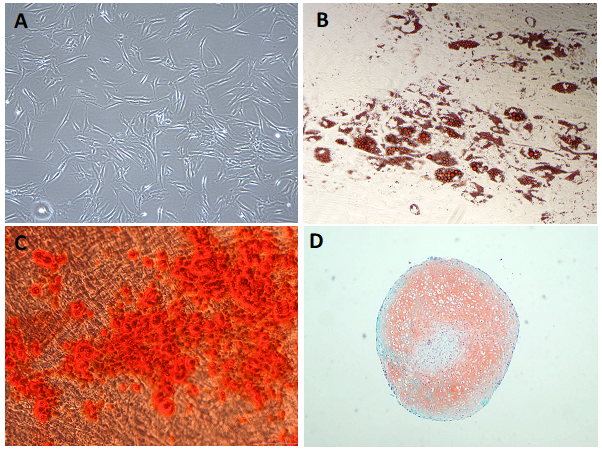
Figure 1. Tri-lineage differenciation of mesenchymal stromal cells (MSCs). A) Undifferenciated MSCs grown in tissue culture flasks in the laboratory showing their characteristic elongated spindle shaped morphology. B) Adipogenesis differentiation: Oil red O dye stains the lipids produced by MSC-derived adipocytes (fat cells). C) Osteogenesis differenciation: Alizarin Red S staining of calcium deposits (red) in mineralised MSC-derived osteoblats (bone cells). D) Chondrogenesis differenciation: Safrin O stain (orange) and Fast Green FCF stain (green) sating for MSC derived chondrocytes (cartilage cells).
MSCs are also known to release a wide range of signalling molecules. Some of them communicate with other cells in the body (Fig. 2). These released molecules have shown to have pro-regenerative effects: modulating the immune system, inhibiting cell death and fibrosis, stimulating vascularisation, and promoting tissue remodelling and repair. The capacity to release these therapeutic molecules underlies the theory that these cells could be used as Medicinal Signalling Cells (giving a new name for these cells but keeping the acronym of MSCs). Scientists are currently studying the use of MSCs and their secreted molecules as potential novel therapies for treating many disease conditions such as immune-mediated disorders as well as chronic diseases.
Finally, several claims have been made that MSCs can be transplanted from one patient to another (also called ‘allogeneic transplantation’) without risk of triggering a severe immune rejection by the host body, like the kind that can occur after an organ transplantation. This property is quite unique to MSCs and has supported the idea that allogeneic MSCs could be used as ‘universal donors’ in the clinic. However, several studies have shown that allogeneic MSCs can indeed induce an immune memory response. This means that the immune system does not mount a response against the MSCs, but still learns to recognise them as ‘non-self’. Further research is still needed to understand how the body would react after a second transplantation of MSCs.
MSCs were originally found in the bone marrow (Fig. 2). The bone marrow contains many different types of cells. Among them are blood stem cells (haematopoietic stem cells; HSCs) and a variety of different types of cells which are collectively called ‘mesenchymal’ cells. Only about 0.001-0.01% of the cells in the bone marrow are MSCs. Nowadays, it is relatively easy to obtain mesenchymal cell types from adult bone marrow for research. However, an increasing number of studies have revealed that MSCs are indeed a highly heterogeneous (mixed) cell population containing cells of different multipotential properties.
For instance, not all the MSCs may have tri-lineage differentiation potential (the ability to differentiate into bone, cartilage and fat, seen in Figure 1) For example, some cells in the mixture may be able to form bone or fat tissues, but not cartilage.
There have since been many claims that MSCs can be found in nearly all tissues, mostly located in perivascular niches (e.g. close to blood vessels). Perivascular MSCs have also been named pericytes. These are known to encircle endothelial cells in the capillaries and microvessels to stabilise them. Some other examples of adult tissue where MSC-like cells have been isolated are peripheral blood tissues, adipose (fat) tissue, dental pulp, and a variety of neonatal tissues (e.g. umbilical cord, cord blood, placenta and amnion tissue), among others (Fig. 2).
However, it has not yet been established whether the cells taken from these other tissues are really the same as, or similar to, the MSCs isolated from the bone marrow. While many studies have claimed that these cells exhibit very similar properties, other studies suggest that they vary depending on the tissue of origin.
The challenge nowadays is to understand the extent of the differences between MSC preparations. This means accounting for variability in the tissue origin, donor-related characteristics (e.g. age, gender, disease) but also variability encountered due to preparation in the lab (ex vivo culture conditions; e.g., isolation procedures, culture media, reagents or media supplements, cell expansion devices, and population doublings) and delivery strategies (e.g. fresh or frozen product, transportation media, injection route).
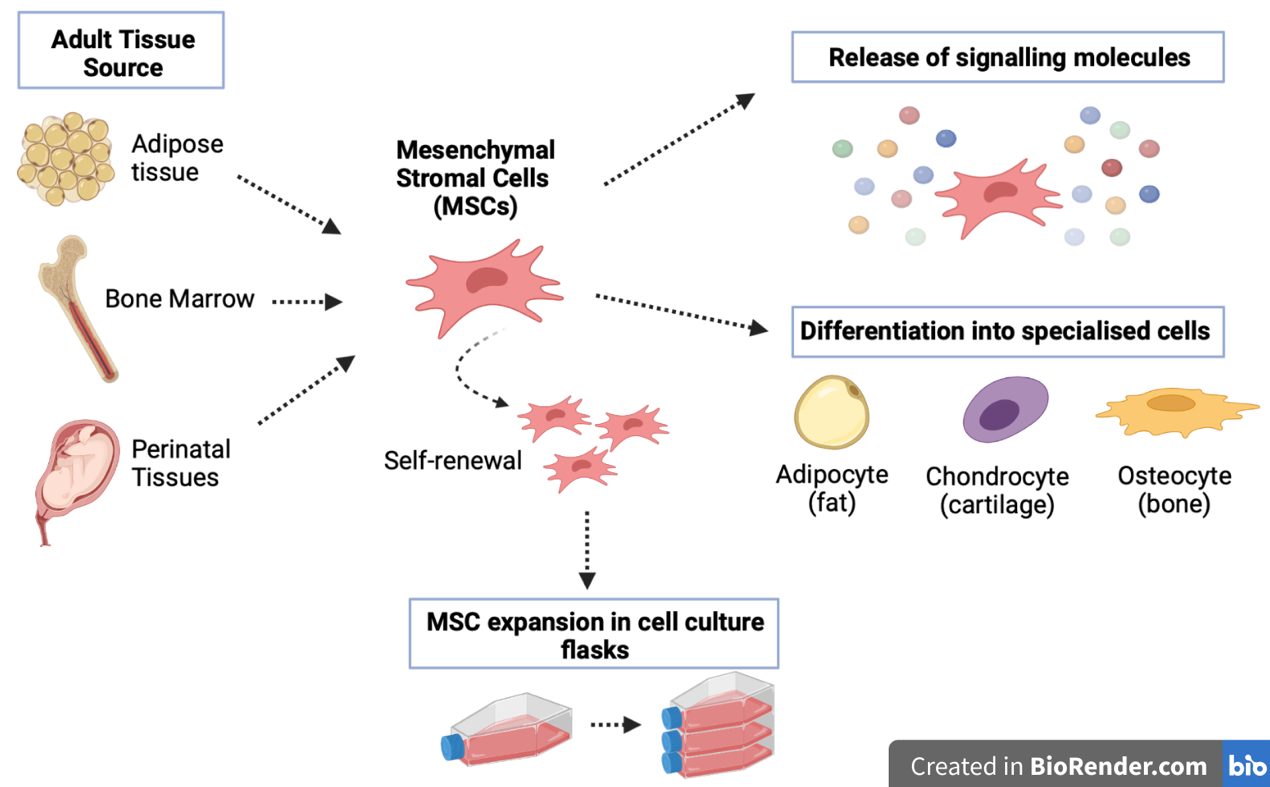
Figure 2. Mesenchymal stromal cell properties. MSCs can be obtained from adult tissues such as bone marrow, adipose (fat) tissue and perinatal tissues (e.g. umbilical cord or placental tissue). MSCs have self-renewal allowing expansion of these cells in the laboratory which allows obtaining large therapeutic doses to use in the clinic. MSCs have shown to be able to make fat, cartilage and bone cells, but they have not convincingly been proven to make other types of cells of the body. MSCs can also release a wide range of signalling molecules that can contribute to tissue remodelling and repair.
Developing new treatments
Several MSC products have been granted regulatory approval by various regulatory agencies worldwide worldwide. For instance, in March 2018, the company TiGenix NV (currently Takeda Pharmaceutical) received the approval by the European Medicines Agency (EMA) for the first allogeneic adipose derived MSC product to treat complex perianal fistulas in adult patients with nonactive/mildly active luminal Crohn’s disease in Europe. MSC treatment have also secured regulatory approval to treat children with GvHD in Canada and New Zealand (Mesoblast Ltd, 2012) and Japan (JCR Pharmaceuticals, 2015) and to treat critical limb ischaemia in India (Stempeutics Research, 2016).
Several possible treatments are currently in early-stage clinical trials for a wide range of conditions.
Bone and cartilage repair
The ability of MSCs to differentiate into bone cells called osteoblasts has led scientists to investigate their use in repairing localised bone defects. These are currently in early-stage clinical trials, investigating the safety of such an approach.
Other research focuses on using MSCs to repair cartilage. Cartilage covers the ends of bones and allows one bone to slide over another at the joints. It can be damaged by a sudden injury like a fall, over a long period by a condition like osteoarthritis, or by life-long ‘wear and tear’. Cartilage does not repair itself well after damage. The best treatment currently available for severe cartilage damage is surgery to replace the damaged joint with an artificial one. Because MSCs can differentiate into cartilage cells called chondrocytes, scientists hope MSCs could be injected into patients to repair and maintain the cartilage in their joints.
Due to MSCs’ anti-inflammatory and immunomodulatory properties, researchers are also investigating the possibility of using MSCs to treat rheumatoid arthritis. Many hurdles remain before this kind of treatment can become a reality. For example, when MSCs are transplanted, most of them are rapidly removed from the body. Researchers are working on new techniques for transplanting the cells, such as developing three-dimensional structures or scaffolds that mimic the conditions in the part of the body where the cells are needed. These scaffolds will hold the cells and may keep them viable for longer. They may also encourage them to differentiate into the desired cell type.
Heart and blood vessel repair
Some studies in mice suggest that MSCs can promote formation of new blood vessels: a process called neovascularisation. MSCs do not make new blood vessel cells themselves, but they may help with neovascularisation in several ways. For example, they may release signalling molecules that stimulate other cells called endothelial progenitor cells (EPCs) – cells that will develop to form the inner layer of blood vessels. They may alo “guide” the assembly of new blood vessels from pre-existing endothelial cells (those that line the blood vessel), or they may act like pericytes (wrapping endothelial cells in the blood vessels to stabilise them). Studies on animals have led researchers to hope that MSCs may provide a way to repair a type of injury arising from reduced blood flow (ischaemic damage). This can be caused by tissue damage due to a heart attack. Several early-stage clinical trials using MSCs in these patients are currently ongoing.
Inflammatory and autoimmune conditions
It has been suggested that MSCs may be able to slow down the multiplication of immune cells in the body. This could reduce inflammation and help treat transplant rejection and autoimmune or inflammatory diseases.
Currently, many early phase clinical studies are investigating the use of MSCs to treat conditions such as graft-versus-host-disease (GvHD), type 1 diabetes, multiple sclerosis, inflammatory bowel disease, kidney or liver disease, and acute respiratory distress syndrome (ARDS). These studies are in the early stages, and much more evidence is needed to establish whether MSCs could really be used for this kind of application.
Current research challenges and the future
Research into therapies using MSCs is still in its infancy. A great deal more work is needed before such therapies can be used routinely in patients. Questions remain about:
- the mechanism of action of these cells (i.e. understanding how these cells exhibit their therapeutic benefit once transplanted)
- how they behave when transplanted into the body
- how they can be delivered to the right place so that they work effectively, the optimal number of cells in a dose
- the optimal tissue origin to obtain MSCs
- the best route of administration
- how all of these relate to the therapeutic effects for the various clinical applications.
By studying how these cells work and interact within the body, researchers hope to develop safe and effective new treatments in the future.
Next steps
Manufacturing of MSCs for patient use must adhere to specific good manufacturing practise (GMP) guidelines. This is necessary for ensuring reproducibility in the manufacturing process as well as ensuring product quality and, most importantly, safety. However, the large-scale production of MSCs for clinical use is facing several challenges. These are largely related to the heterogeneous nature of MSCs, and to the current lack of standardisation of MSC manufacturing protocols.
Firstly, MSCs have shown to possess an intrinsic variability concerning the tissue origin, donor characteristics (age, sex, disease status), and age in culture. It has yet to be determine which is the ‘optimal’ MSC type to be used in the clinic; indeed, this may depend on the disease to be treated.
In addition to this, there are other ex vivo characteristics related to the manufacturing processes for the large production of clinical doses of MSCs. These also contribute highly to the variability in MSC cultures. Some of the in-process variables include:
- use of different isolation techniques
- culture media and reagents
- cell expansion devices (flasks or bioreactors).
All of these factors have been shown to affect the biological properties of MSCs. One single change in the process of cell manufacturing, for instance changing one component in the cell culture media, has the potential to change the final product. Most of the in-process variables have not yet been standardised across laboratories worldwide. This represents a bottle-neck in the field, as it often impedes the compatibility of MSC safety and efficacy profiles across different clinical studies. Establishing guidelines to ensure standardisation of MSC manufacturing processes is essential, and will result in more optimal production of MSC therapies that can be used widely for patient benefit.
Another current challenge is related to the lack of complete understanding of the mechanism of action (MoA) of these cells (ie. how are these cells conferring their therapeutic benefit once transplanted). Understanding the MoA of MSCs is key for developing a test that can predict the therapeutic efficacy of MSCs before it is transplanted into patients (a potency assay). These tests are also key for ensuring quality, consistency and stability of the MSC product prior it is released into the patients. This test is required in phase 3 clinical testing; therefore it should be developed in earlier phases of preclinical and clinical development of MSC products.
Finally, there is a need to produce more affordable MSC therapies for worldwide use. The standardisation of the manufacturing processes will ultimately increase safety and reproducibility during the manufacturing process, and will reduce production times and manufacturing costs, thus producing more cost-effective therapies.