[Poster] The marketing authorisation of advanced therapy medicinal products under the regulation of the European Union
Abstract
Advanced Therapy Medicinal Products (ATMPs) is a European classification of medicinal products based on genes, cells and tissues specifically regulated in the European Union (EU) from the entry into force of Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on ATMPs. Any company wishing to market ATMPs within the EU must hold a Marketing Authorisation (MA) issued by the European Commission under the “centralised procedure”, after a single application to the European Medicines Agency (EMA) and its scientific assessment by the Committee for Advanced Therapies. The purpose of the MA is to ensure high quality, safety and efficacy for ATMPs, with a positive risk-benefit balance, to be commercialised. The MA is valid in the EU Member States, Iceland, Norway and Liechtenstein for a five-years period.
From the adoption of the EU regulation, 24 ATMPs have been granted a marketing authorisation in the EU and the European Commission decision is pending for another one. There has been a clear increase in approvals from 2018, and most approved ATMPs are gene therapy medicinal products. Almost half of them benefited from expediting pathways or regulatory support schemes for (innovative) medicines to be commercialised in the EU. Nevertheless, MA has been withdrawn or not renewed for 7 of them. This poster will present the European regulatory requirements and pathways for ATMPs to obtain marketing authorisation, and discuss the challenges of their commercialisation.
Poster
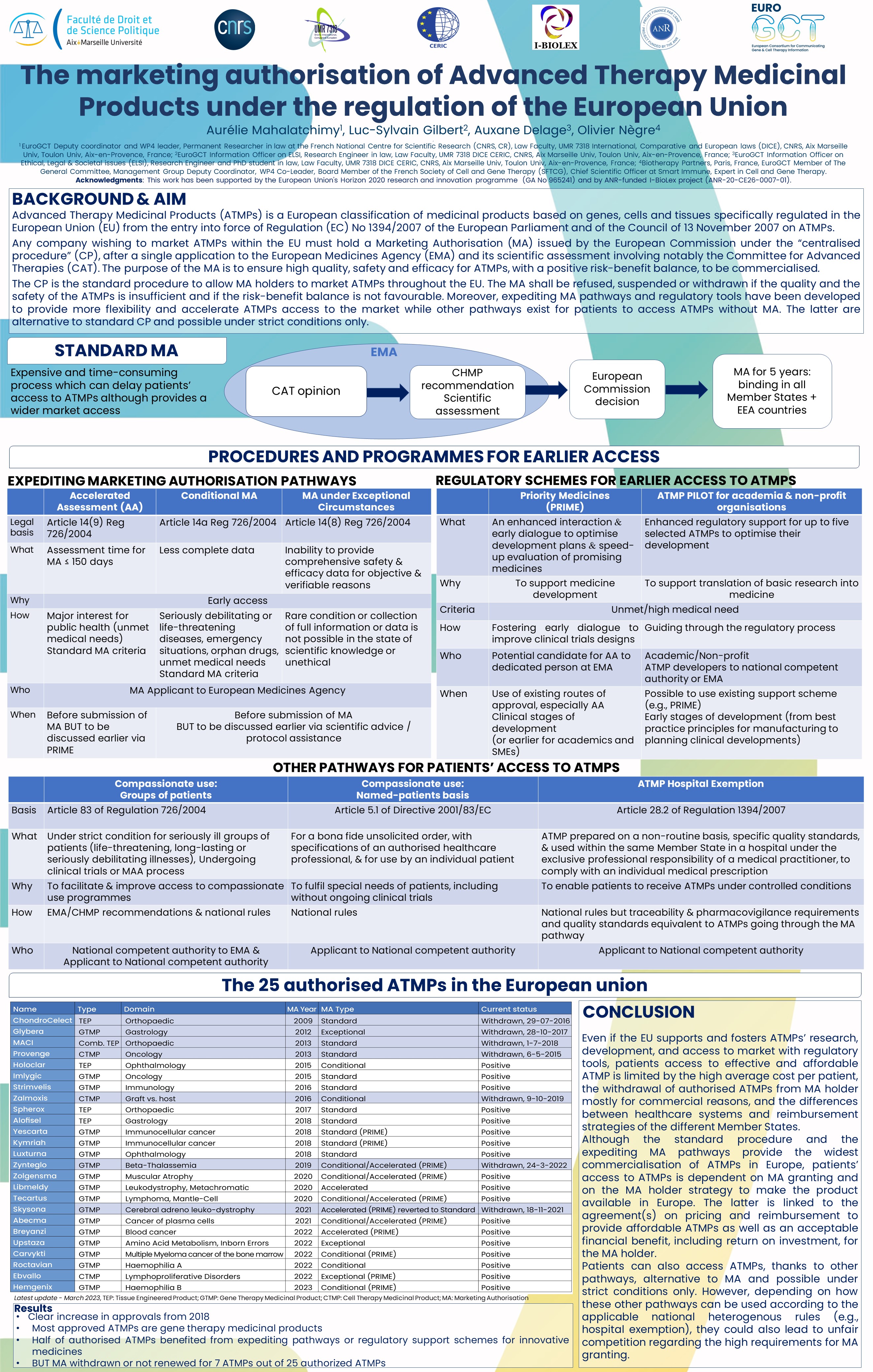
The pdf file of this poster can be downloaded from the attachments section at the bottom of this page
An updated version of the table 'The 25 authorised ATMPs in the EU' from the poster above:
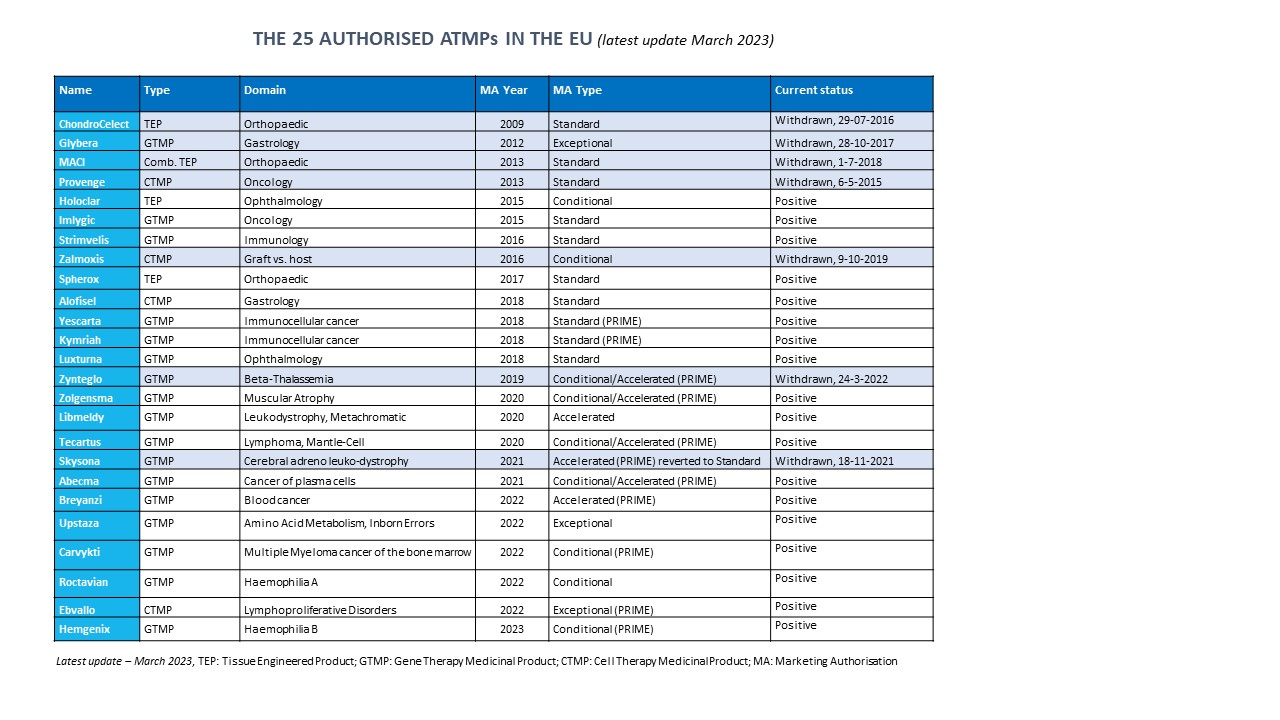
The pdf file of this table can be downloaded from the attachments section at the bottom of this page
Authors
Luc-Sylvain Gilbert, EuroGCT Information Officer on Ethical, Legal & Societal issues, Aix-en-Provence, France
Auxane Delage, EuroGCT Information Officer on Ethical, Legal & Societal issues, Aix-en-Provence, France
Aurélie Mahalatchimy, EuroGCT Deputy coordinator and WP4 leader; UMR 7318 DICE CERIC, Aix-Marseille University, Centre National de la Recherche Scientifique, Marseille, Provence-Alpes-Côte d’Azu, France
Olivier Negre, EuroGCT Member of The General Committee Management Group, WP4 Co-Leader; Board Member of the French Society of Cell and Gene Therapy (SFTCG), Paris, France; Expert in Cell and Gene Therapy at Biotherapy Partners, Paris, France.