About EuroGCT’s Key Resources
The resources developed by the EuroGCT consortium and/or with collaborators has been carefully designed, compiled and curated to meet the various stakeholders’ needs in improved access to practical steps regarding gene and cell therapy development. EuroGCT’s key resource are tagged with a ![]() badge and can be found from the Research Pathways, or accessed through the EuroGCT Key Resources collection. Information on the article’s publication date, last update date, the authors, reviewers and their affiliations are listed in the Acknowledgement section.
badge and can be found from the Research Pathways, or accessed through the EuroGCT Key Resources collection. Information on the article’s publication date, last update date, the authors, reviewers and their affiliations are listed in the Acknowledgement section.
To facilitate the communities’ access to practical information which are not always available in other languages outside of English (e.g. the European Union Guidance), EuroGCT WP4 is working on developing guidance in additional European languages. We will populate the site as more language versions become available.
Each piece of our key resource contains a Research Pathways overview and a table of contents on its right (Fig. 1):
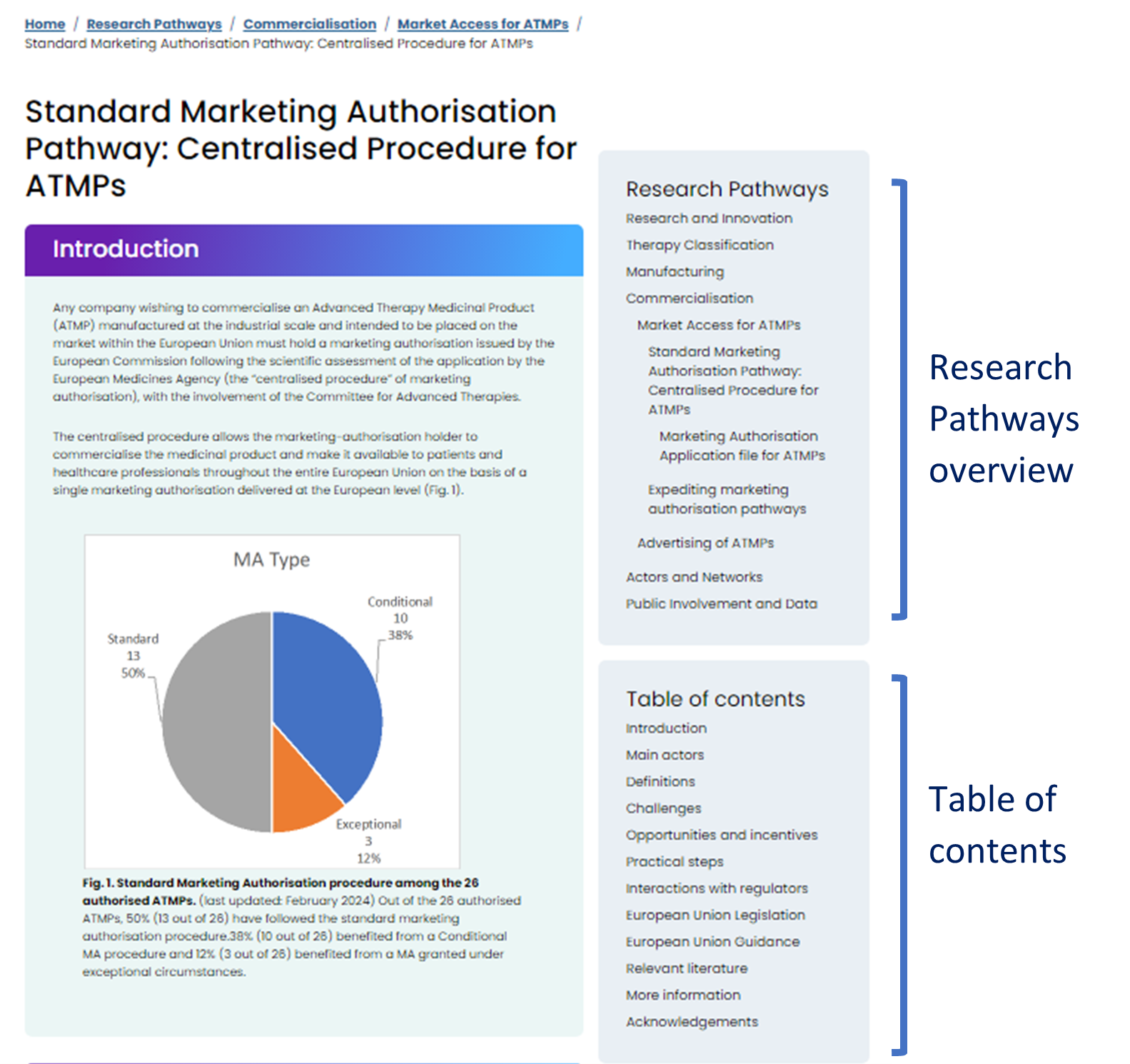
The Research Pathways overview shows the information structure and provides the links to relevant parent and daughter entries. The table of contents shows the sections contained within the entry. Below we will explain in detail.
Research Pathways Overview / Themes
The EuroGCT key resources are designed and organised to capture the various stages GCT stakeholders may encounter in the processes of bringing a therapy from lab to patient.
The detailed information structure is laid out in the Research Pathways Draft tree (Fig. 2). The “Themes” filter of the Research Pathways database is also based on this draft tree.
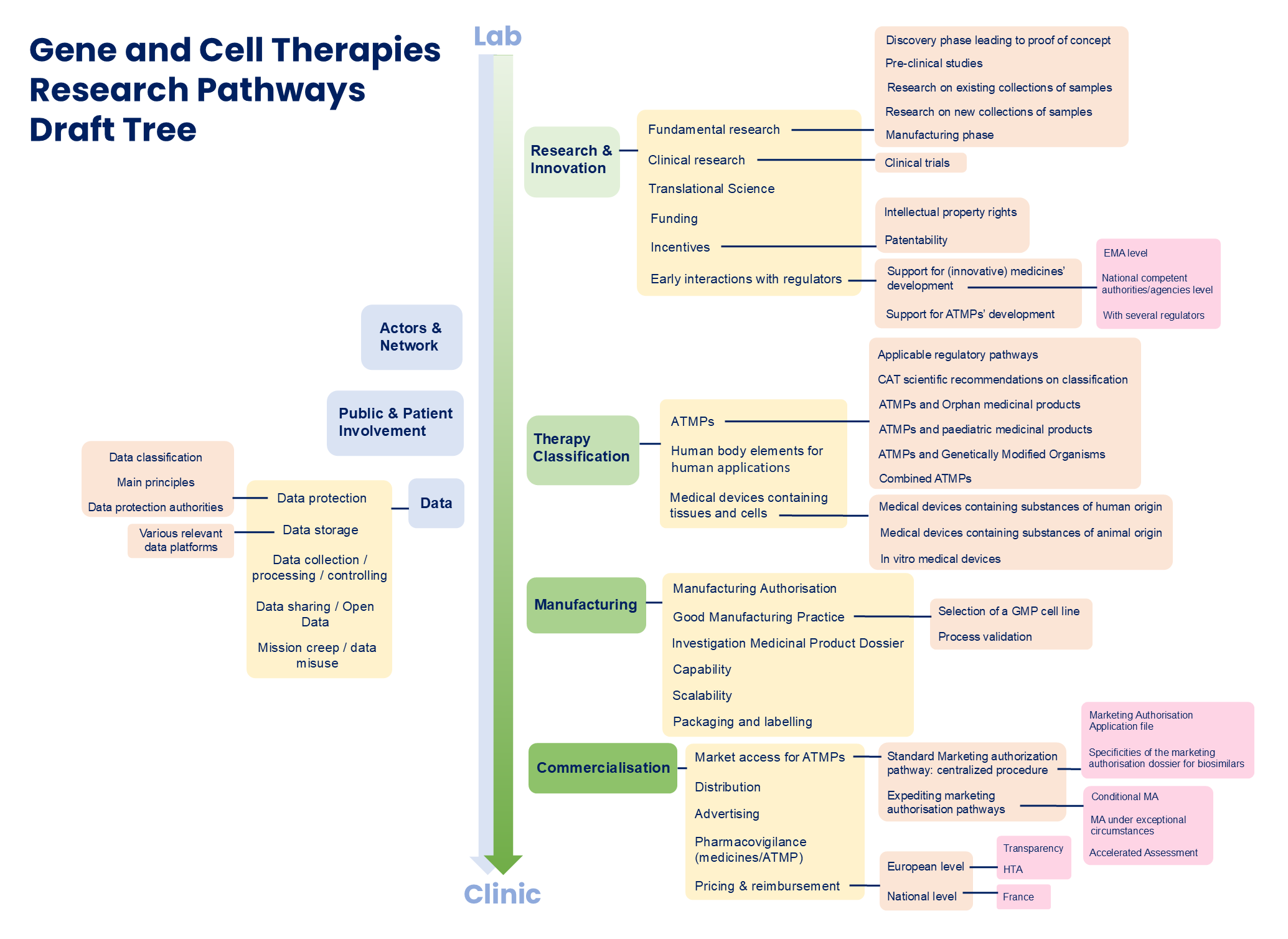
Briefly, the lab to patient process is grouped into six main stages:
- Research and Innovation: this section explores the steps from fundamental to pre-clinical and clinical research underpinning the development of innovative gene and cell therapies. It also covers fundings, incentives and early interactions with regulators.
- Therapy Classification: therapy classification determines the legal mechanisms that govern the regulation of GCTs. How different types of GCTs are regulated in the EU is described here.
- Manufacturing: the manufacturing section concerns the industry-scale production of CGTs for supporting clinical development and commercialisation.
- Commercialisation: the commercialisation section covers marketing authorisation, and post-authorisation activities such as pharmacovigilance, pricing and reimbursement.
- Actors and Networks: the actors and networks section maps the stakeholders and their activities in Europe and beyond.
- Public Involvement and Data: this section introduces best practices for public involvement and the handling of health data throughout the GCT development processes.
Table of content / Sections in each entry
Each piece of EuroGCT’s key resources is accompanied by a table of contents on the right-hand side. Each fully completed entry contains the following sections:
- Introduction: what happens at this stage, and what do we know about it.
- Main actors: the main players involved during this stage.
- Definitions: the languages and terms the stakeholders are speaking of, and their definitions.
- Challenges: the legal, ethical, and societal challenges.
- Opportunities and incentives: the opportunities, supports, incentives (e.g. scientific advice, designations) existing at this stage.
- Practical Steps: detailed practical steps as well as existing links relevant for this sub-entry.
- Interactions with regulators: the existing mechanism(s) of interaction with regulators, and gaps/needs of interaction with regulators.
- European Union Legislation: the applicable European Union legislation with direct link to the full text and to existing documents’ summary as provided on the official European Union law database, Eur-Lex, in many EU languages.
- European Guidance: the applicable European Guidelines with direct link to the history of the guidelines (where available), which includes the link to the current effective version or directly to the full text of the current effective version.
- Relevant Literature: a list of literature relating to the topics covered by the sub-entry with link to the existing scientific abstract or to the full text if available in open access. This list of literature is based on a thorough research on the databases listed here.
- More: any other information and links that are considered to be relevant to the sub-entry concerned.
- Acknowledgements: information on the article’s publication date, last updated date, the authors, reviewers and their affiliations
Published: 05/06/2024
Last updated: 26/06/2024

